Ennov RIM - Regulatory Information Management Software
Gain control of your product details, registrations, submissions and regulatory activities
- Comprehensive RIM solution
- Intrinsically connected to Ennov Doc and Ennov Dossier
- Fully configurable to support your entire product portfolio
- Workflow driven process automation
- Robust data querying, reporting and visualization
- Fully compliant xEVMPD submissions
The Regulatory Information Management Challenge
The global regulatory landscape is getting more complex. In the United States, half of the FDA’s NDA approvals come with a commitment to conduct additional clinical trials. In the European Union, the complexity of regional submissions continues to increase. And, while emerging markets represent growth opportunities, the vague submission requirements of local health authorities can make gaining market approval somewhat unpredictable. All of these factors underscore the need for robust Regulatory information management capabilities.
Regulatory information management at global organizations is rarely integrated and usually consists of collections of spreadsheets, homegrown databases, emails and ad hoc reports—each full of redundant and/or inconsistent data.
However, as product registrations become increasingly complex and volumes increase, companies are realizing that the ability to answer business-critical questions about all regulatory activity in an efficient and timely manner is critical to effective operations that ensure enterprise-wide compliance.
Ennov RIM - Your Single Authoritative Source of Regulatory Information
Imagine all of your regulatory information regarding products, registrations, submissions, correspondence and commitments in one centralized place accessible from anywhere. With Ennov RIM, life sciences companies can streamline their regulatory processes, improve their data quality, quickly answer business-critical questions and effectively respond to health authority requests.
Ennov RIM is a purpose-built application (based on Ennov Process) for the management and tracking of therapeutic product details and registration information. Whether you are planning the launch of a new product or handling variations to existing registrations, Ennov RIM provides Regulatory personnel with the key information and functionality to effectively manage your product portfolios worldwide.
The 100% web-based solution includes an intuitive and configurable user interface, Regulatory task management, e-mail notification capabilities and correspondence and commitment tracking functions. Ennov RIM’s Regulatory activity planning feature provides the ability to manage submission projects that span many applications – information is entered once and replicated across products, countries and applications as appropriate.
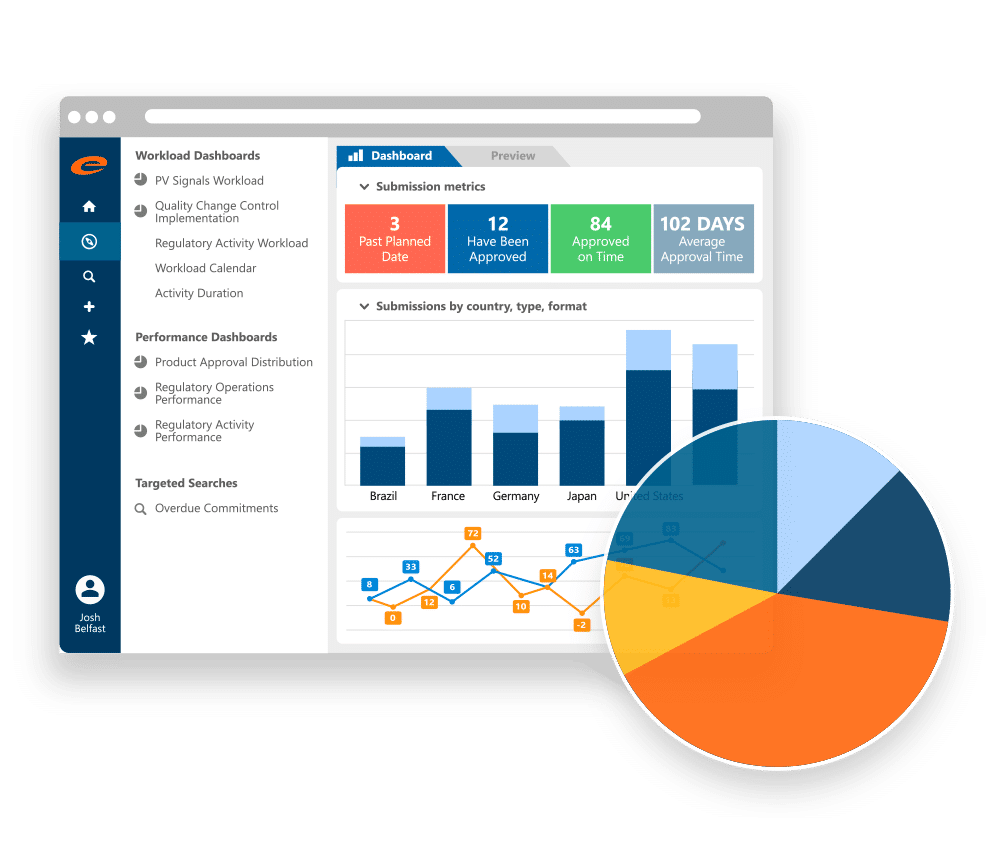
Increase Operational Efficiency
Ennov RIM translates data into action. It is a process-driven solution that clearly identifies what needs to be done, when and by whom. Its robust workflow capabilities ensure visibility into assignments and deadlines through automatic e-mail notifications and real-time status updates. Robust querying and visual dashboard reporting provide complete visibility into the all the activities within your Regulatory operation.
Ennov RIM provides the ability to efficiently manage your entire product portfolio including small molecule therapies, large molecule therapies, medical devices, drug-device combinations, nutritionals/supplements and more. Our comprehensive yet flexible solution will give you confidence that your registrations stay up-to-date and all of your products stay on the market.
Fully Compliant xEVMPD Submissions
Ennov RIM includes automated, compliant and efficient submissions to XEVMPD. Messages are compiled, validated and can be submitted through Ennov RIM. Our integrated workflows ensure that XEVMPD compliance can be achieved and maintained with ease.
Innovative Dashboards to Support both Planning, Impact Analysis and Metrics
Ennov’s RIM dashboards support all of the professionals who use RIM. Regulatory Operations can see the volume of upcoming workload, and instantly understand coming due and overdue activities. Quality professionals can perform impact analysis to see what registrations are affected by a change in manufacturer, active substance or excipient. Management can view information about timeliness and volume of regulatory activities. Out of the box dashboards cover all regulatory activities including submissions, correspondence and commitments and provide specialized analysis for PSURs, impact analysis, and much more.
Core Capabilities
- Centralized management of detailed product information
- Market authorization and registration management
- Submission and Regulatory activity planning and tracking
- Automatic linkage between substances, products, registrations, activities, dossiers, submissions and documents
- Correspondence and commitment tracking
Key Features
- Role-based access and action rights
- Configurable data model
- Automated email notifications
- Intuitive user interface
- Robust workflow engine
- 21 CFR Part 11 compliant
The Impact of Ennov RIM at Foghorn Therapeutics
Efficient regulatory information management can be complex. Your software shouldn’t be.
Aguettant Case Study
“It’s really nice to work with a software provider that shows a great deal of innovative spirit, has great ambition and is proactive. Above all, I really appreciate Ennov placing particular emphasis on the customer’s user experience.”
Cyrille Jeune,
Regulatory Affairs Systems Manager

Septodont Case Study
“With more than 1500 MAAs in 150 countries, we face a real productivity challenge. With Ennov, we have been able to issue 400 dossier in just 18 months. For the first time, our users are experiencing tremendous time savings when locating documents.”
Aurélie Becquet,
Regulatory Affairs
Regulatory
World-Class Regulatory Content and Information Management
The Ennov Regulatory suite has the power and flexibility to support the entire regulatory product lifecycle from the early planning of registration targets through to product retirement. It is an invaluable solution for regulatory activity planning, product registration management, dossier creation, dossier management and more.
Regulatory
World-Class Regulatory Content And Information Management
The Ennov Regulatory suite has the power and flexibility to support the entire regulatory product lifecycle from the early planning of registration targets through to product retirement. It is an invaluable solution for regulatory activity planning, product registration management, dossier creation, dossier management and more.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.
