Ennov Regulatory Suite
World-class Regulatory content and information management
A unified, centralized, end-to-end solution, robust enough to support the regulatory information workflow from the earliest stages of research and development to beyond market authorization, is essential for companies striving to achieve regulatory operational excellence. Companies in the life sciences industry (whether pharma, biotech, med device, or animal health) understand that a unified regulatory solution helps drive harmonization, promote standardization, improve collaboration, ensure compliance, eliminate waste, reduce costs, accelerate product release, and compete more effectively in global markets.
The Ennov Regulatory Suite has the power and flexibility to support the entire regulatory product lifecycle from the early planning of registration targets through to product retirement. The Ennov Regulatory suite is an invaluable tool for regulatory activity planning, product registration management, dossier creation, dossier management and more.
Benefits
- A single authoritative source
Manage and track all Regulatory documentation, dossiers, and data within a single application to streamline operations and increase efficiency. - Improved performance
Eliminate manual, paper-based processes and record keeping. Automate repetitive and error-prone tasks to achieve productivity gains.
- Global connectivity
Foster collaboration across organizational and geographic boundaries with a unified, harmonized solution that features an intuitive user interface with local language capabilities. - Increased visibility
Gain valuable insights through our AI-powered dashboard to identify and prevent problematic trends before they lead to quality issues.
Come join more than 450 Life Sciences companies around the world being powered by Ennov
The Impact of Ennov RIM at Foghorn Therapeutics
Efficient regulatory information management can be complex. Your software shouldn’t be.
Aguettant Case Study
“It’s really nice to work with a software provider that shows a great deal of innovative spirit, has great ambition and is proactive. Above all, I really appreciate Ennov placing particular emphasis on the customer’s user experience.”
Cyrille Jeune,
Regulatory Affairs Systems Manager

Septodont Case Study
“With more than 1500 MAAs in 150 countries, we face a real productivity challenge. With Ennov, we have been able to issue 400 dossier in just 18 months. For the first time, our users are experiencing tremendous time savings when locating documents.”
Aurélie Becquet,
Regulatory Affairs
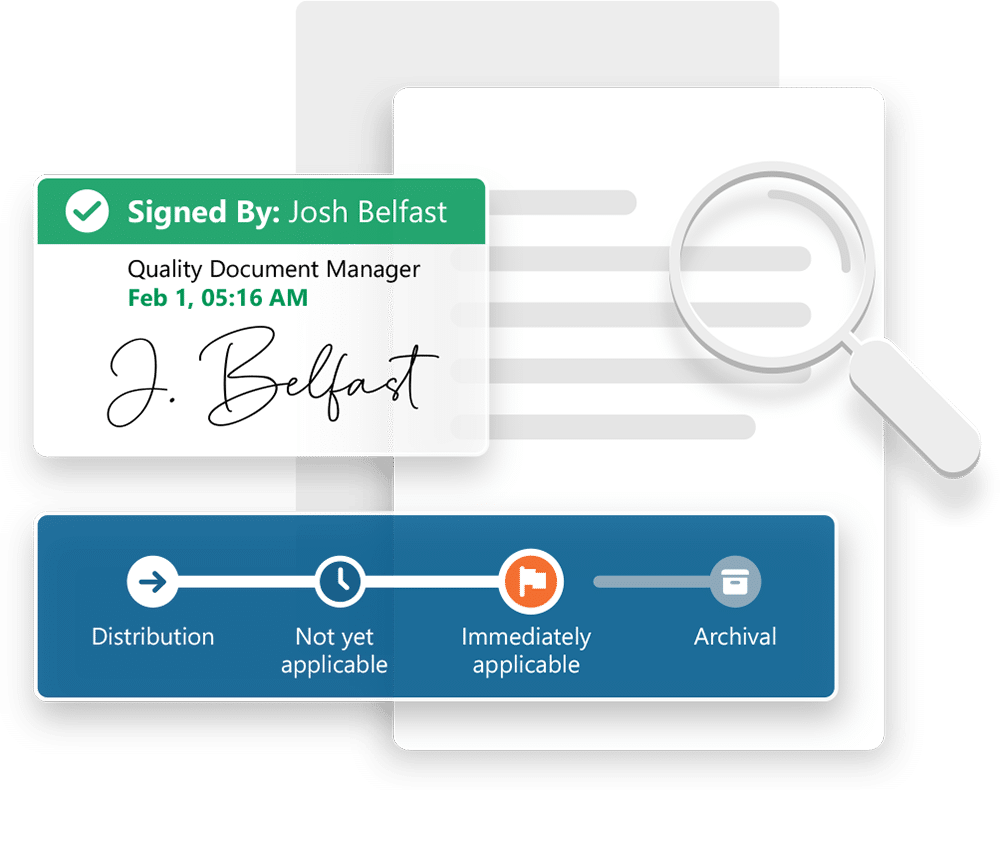
Ennov Doc for Regulatory
Effectively and efficiently manage Administrative, Non-Clinical, Clinical and CMC documentation with Ennov Doc.
- Full featured EDMS
- Highly configurable document life cycles
- Metadata-based document model
- Easy and intuitive searching
- Scalable and secure
Ennov Dossier
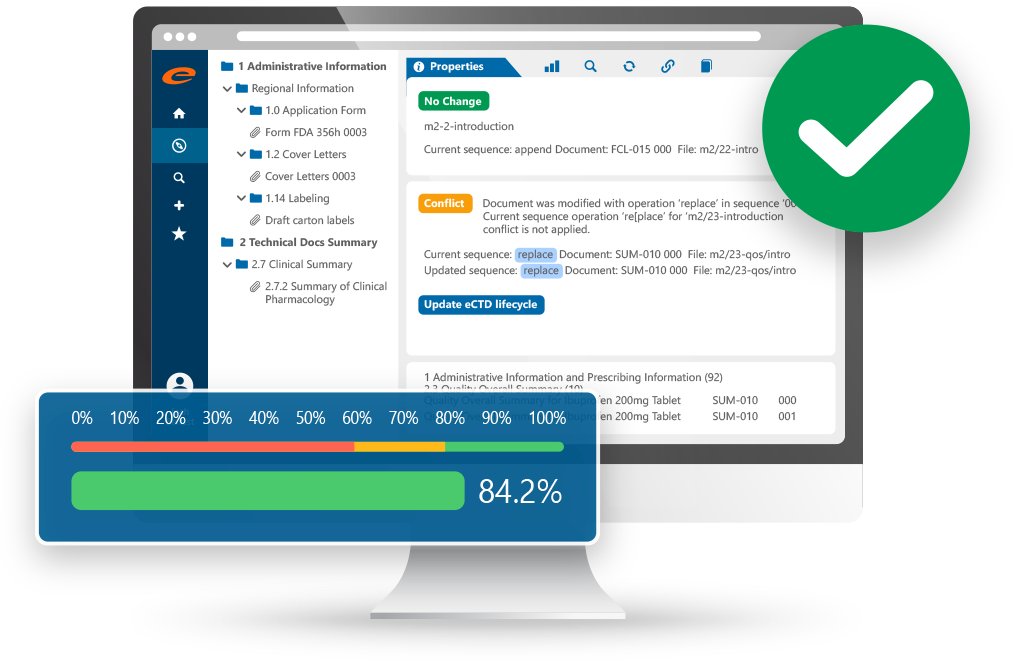
Accelerate approvals with our intuitive and compliant Submission Publishing software.
- Create, manage and publish Regulatory submissions
- Publish to any output format
- Robust hyperlinking and bookmarking
- Built in validator ensures compliant submissions
- 100% web-based, ideal for global deployments
Ennov RIM
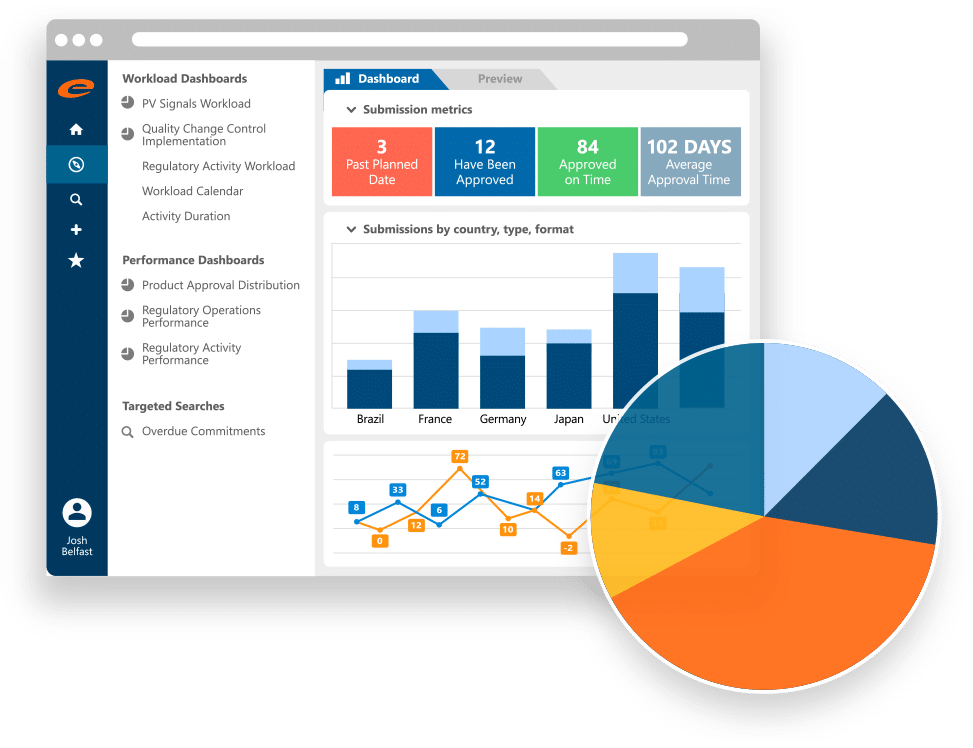
Gain control of product details, registrations, submissions and regulatory activities with our robust Regulatory Information Management software.
- Comprehensive RIM solution
- Intrinsically connected to Ennov Doc and Ennov Dossier
- Fully compliant xEVMPD submissions
- Fully configurable to support your entire product portfolio
- Workflow driven
- Robust data querying, reporting and visualization
Ennov IDMP
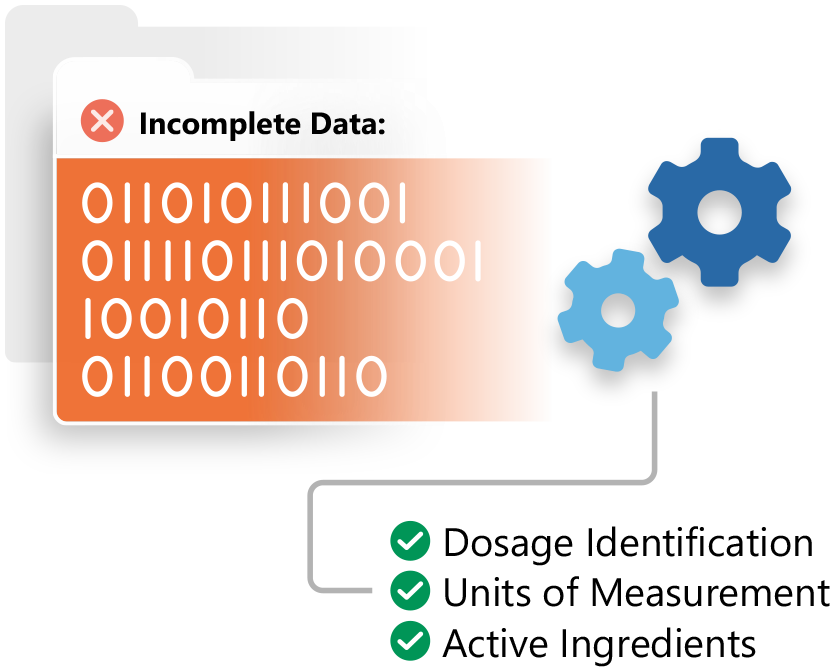
Prepare your organization for the emerging ISO standards and health authority requirements with Ennov IDMP.
- Simplified IDMP management
- Integration with the EMA’s PMS
- Increased data quality
- IDMP services
- Works with any RIM system to ensure IDMP compliance
DocShifter
Automate PDF conversion, enrichment and quality control and accelerate regulatory document preparation.
- Generate submission-ready PDFs from any DMS & RIM
- Automate report level publishing
- Check and fix your PDFs to ensure regulatory compliance
- Check and fix your Word documents to ensure regulatory compliance
- Future-proof your digital documents for archiving


Ennov Artwork
Simplify artwork management and accelerate time to market with a centralized, compliant, and collaborative solution.
- Centralized repository with versioning, annotation, and comparison tools
- Integrated with Regulatory, Quality, Submission, and Supply data
- Workflows and dashboards streamline approvals and reduce delays
- Compliant with 21 CFR Part 11 and EU GMP Annex 11 for peace of mind
