Ennov Doc for Quality
Enterprise Quality Document Management Software
Manage and control GxP documents effectively and efficiently with Ennov Doc
- Full featured EDMS
- Configurable document life cycles
- Metadata-based document model
- Easy and intuitive searching
- Scalable and secure
- 21 CFR Part 11 compliant
The Quality Document Management Challenge
Managing and sharing controlled documents effectively and securely in a global environment is a challenge. Highly regulated companies like those in the biopharmaceutical industry are required to manage and track documentation per GxP and ISO standards. These quality standards require proof of document creation, editing, review, approval and issuance.
Storing documents on file shares across disparate locations is inefficient, impedes productivity and introduces risk. Personnel often needlessly spend time hunting for the correct version of a document – prolonging their tasks at hand and increasing their frustration.
If these challenges sound all too familiar, our comprehensive full-featured Enterprise Document Management System, Ennov Doc for Quality, is a quality management software that can help you streamline processes, ensure compliance and increase operational efficiency.
Unified Access to Quality Documents
Ennov Doc for Quality provides a comprehensive solution to managing GxP documentation. The document inventory is pre-configured in alignment with the DIA GMP Reference Model and includes all required document categories, groups, sub-groups and artifacts.
Ennov Doc’s metadata-based document model provides the flexibility to adapt this model to your company’s organizational needs. Our intuitive suite of design utilities allow administrators to configure and manage the system without needing IT skills.
Ennov Doc’s scalability and security enables you to safely manage large volumes of documents – making it the perfect solution for global deployments.
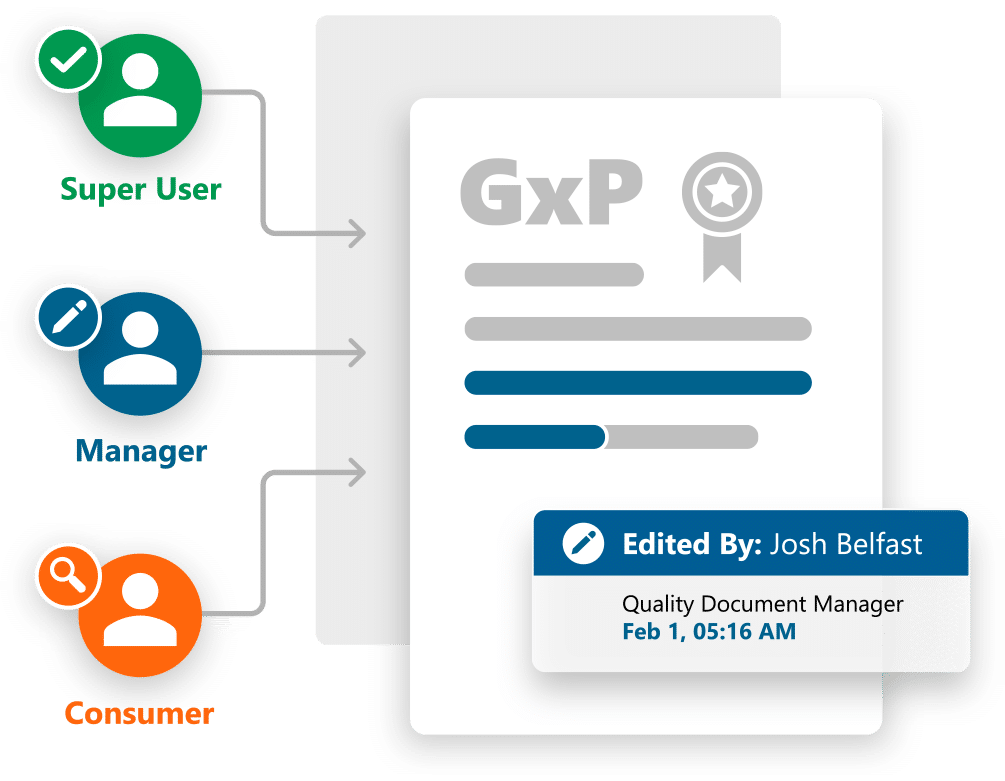
Improved Productivity and Efficiency
Ennov Doc’s intuitive user interface and efficient search capabilities allow every employee to quickly locate and access the documents they require. The user-centric design and connectivity to Microsoft Office 365 and Google Drive improves user adoption, promotes collaboration and accelerates your return on investment.
Ennov Doc provides instant access to documents without requiring MS Office or Acrobat to be installed on the desktop. The integrated PDF Viewer increases security by providing read-access to documents from within application – eliminating the need to download an uncontrolled copy.
Automatic periodic reviews, integrated change control, robust revision management, controlled printing and electronic signature manifestation functionality make Ennov Doc for Quality the perfect solution to ensure GxP and ISO compliance.
Manage All Quality Documents
Our customers use Ennov Doc Quality to support a wide variety of GxP document types including governance, procedures, manufacturing, quality, audit, validation, packaging and more. Ennov Doc’s high degree of configurability and seamless integration with our Business Process Management System (Ennov Process), our composite document and publishing system (Ennov Dossier) and our data visualization and reporting tools (Ennov Analytics) allows them the flexibility to meet their corporate quality standards.
As an added benefit, Ennov Doc fully complies with FDA’s 21 CFR part 11 requirements (electronic signature, audit trail, records management), making this quality document management software a perfect fit for regulated industries such as pharmaceutical, biotechnology, animal health, medical device and others.
Core Capabilities
- Pre-configured document inventory
- Advanced life cycle management
- Flexible rights management
- Automatic PDF rendering and tag management
- Full text and metadata based searching
- Controlled printing, copy and paste
- Periodic review, expiration and archive management
- Office 365 and Google Drive connectivity
Key Features
- Integrated work list dashboard
- Configurable document types, workflows and views
- Automated email notifications
- Intuitive user interface
- Integrated PDF Viewer
- Composite document support
- 21 CFR Part 11 compliant
- 100% web-based
Pharmacosmos Case Study
“It makes our daily handling and signing of documents much faster, reduces the use of paper and eliminates signing documents by hand.”
Flemming Simonsen,
Director, GxP System Compliance Pharmacosmos
Quality
A Comprehensive QMS to Improve Efficiency and Ensure Compliance
Ennov Quality provides a predefined inventory of quality documentation, processes and workflows that are based on accepted industry standards and best practices. This allows your organization to get your QMS system into production and begin realizing your return on investment more quickly. Ennov Quality, like all Ennov solutions, is easy to configure and requires no IT skills.
A Comprehensive QMS to Improve Efficiency and Ensure Compliance
Ennov Quality provides a predefined inventory of quality documentation, processes and workflows that are based on accepted industry standards and best practices.
This allows your organization to get your QMS system into production and begin realizing your return on investment more quickly. Ennov Quality, like all Ennov solutions, is easy to configure and requires no IT skills.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.
