Ennov PV
Reporting & Data Analysis
Compliant by default. Fast by design. Global by nature.
- Generate data extracts, paper reports, and XML submissions with ease
- Analyze safety data using pre-supplied or custom-built queries
- Fully configurable workflows to support end-to-end case processing
- Seamlessly integrates into existing pharmacovigilance operations
- Trusted by pharma, CROs, service providers, and regulators
- Supports all standard single case and aggregate reports
Report
Next Generation Safety Reporting
Create and submit Adverse Event case reports with a next generation solution. This 100% web-based solution that leverages an elegant and intuitive user interface that instantly generates:
- Paper report outputs such as CIOMS and MedWatch 3500s.
- Electronic adverse event reports in full compliance with the ICH E2B standard (R2 and R3 formats).
- Aggregate listing outputs like PSUR, DSUR, and PBRER
The solution also includes powerful querying capabilities which allows business teams to monitor case handling, track compliance, and execute in-depth trend analysis. Advanced data field linking supports context linked queries. Enhanced grid presentation outputs with multi-row options for multi-record data takes interpreting data to the next level.
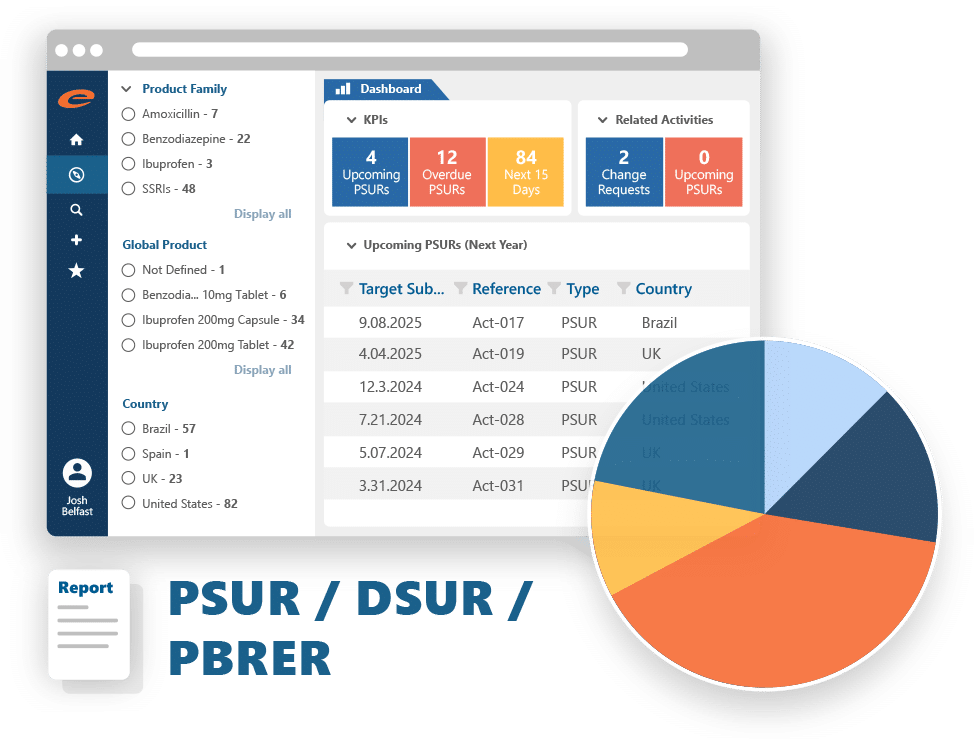
Core Capabilities
- Elegant, easy-to-use interface
- Advanced query tool allowing the creation of complex custom queries with additional search operators and logic
- Full support for search wildcards and removal of case sensitivity constraints
- Advanced data field linking to support context linked queries
- Enhanced grid presentation outputs – with multi-row options for multi-record data
- Ability to save and share layouts
- Ability to export parameters and layout definitions
- Standard regulatory extractions – such as PSUR
Key Features
- Dramatically speeds up report generation
- Modern and intuitive layout
- Reduced training overhead to accelerate on-boarding
- Seamless connectivity to PV-Works or PV247
- 100% web-based
- 21 CFR Part 11 compliant
Pharmacovigilance
An End-To-End Solution for Collecting, Reporting and Analyzing Human and Vet PV Data
The Ennov Pharmacovigilance solution keep the collection, management, assessment, and reporting of human or veterinary adverse events in one unified database while also providing advanced signal detection and PV data analysis tools.
An End-To-End Solution for Collecting, Reporting and Analyzing Human and Vet PV Data
The Ennov Pharmacovigilance solution keep the collection, management, assessment, and reporting of human or veterinary adverse events in one unified database while also providing advanced signal detection and PV data analysis tools.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.
