Ennov DataLabs EDC
Transform your clinical trials with Ennov DataLabs: Reliable. Flexible. Efficient.
- Focus resources effectively with Targeted Source Data Verification (SDV)
- Seamlessly integrate with other systems through robust APIs
- Enhance patient safety with real-time safety reporting
- User-friendly interface for rapid study builds
- 100% web-based solution
Introduction
Ennov DataLabs EDC revolutionizes clinical data management, providing a flexible, reliable, and efficient electronic data capture (EDC) system. Designed for biopharmaceutical sponsors, CROs, and medical device manufacturers, Ennov DataLabs ensures superior usability and functionality, supporting the entire lifecycle from study design to data reporting.
The Challenge of Clinical Trial Management
Clinical trial management is fraught with complexities, from meeting stringent regulatory requirements to managing vast amounts of data. Organizations often struggle with maintaining data quality, ensuring patient safety, and optimizing resource allocation. Inefficiencies in trial management can lead to increased costs, delays, and potential risks to patient safety. Without a robust EDC system, these challenges become magnified, impacting the overall success and reliability of clinical trials.
Solution Overview
Ennov DataLabs addresses these challenges head-on with a comprehensive suite of advanced features and capabilities.
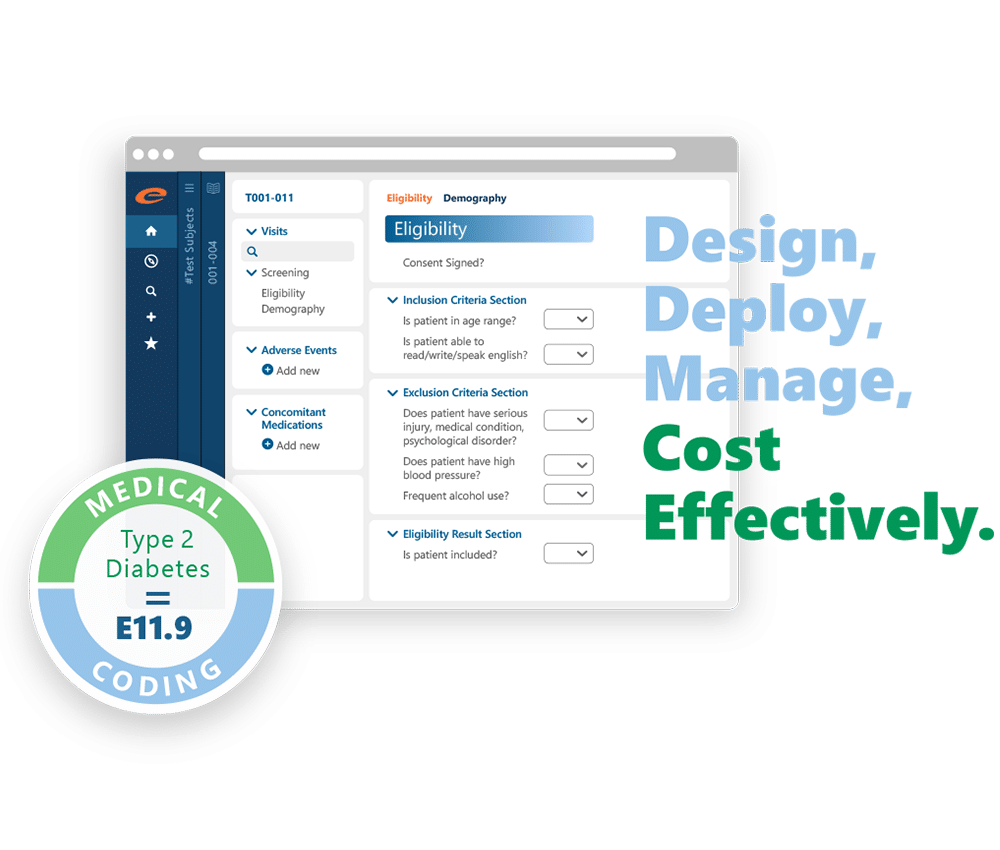
It stands out with its targeted Source Data Verification (SDV) that focuses monitoring efforts where they are most needed, reducing on-site monitoring activities and associated costs. This enables study teams to make data-driven decisions, enhancing efficiency and resource allocation.
Advanced Analytics and Reporting
The platform offers robust reporting tools, including dashboards and ad-hoc reporting capabilities, providing clear visibility into trial metrics and aiding strategic decision-making. Integrated safety reporting ensures real-time notifications of critical safety events, enhancing patient safety and compliance.
Implementation and Integration
With a simple user interface and drag-and-drop study build features, Ennov DataLabs facilitates rapid and intuitive study designs. Its robust API web services enable seamless integration with various systems, ensuring comprehensive data management and eliminating discrepancies.
Core Capabilities
- Targeted Source Data Verification (SDV)
- Real-time safety reporting
- Seamless integration via robust APIs
- Intuitive study build tools
- Comprehensive data management and reporting
Key Features
- Centralized repository for study design objects
- Built-in edit checks for data validation
- Drag-and-drop interface for study builds
- Dynamic generation of patient-specific CRFs
- Visual comparison of study design changes
- Web-based with 21 CFR Part 11 compliance
Clinical
A Comprehensive Solution to Capture and Manage Clinical Trial Information
The Ennov Clinical suite consists of Clinical Data Management applications (EDC, RTSM and ePRO) as well as Clinical Trial Management applications (CTMS and eTMF) that are available for deployment in the cloud or on premises.
Efficiently & Securely Capture & Manage Clinical Trial Information
The Ennov Clinical suite consists of Clinical Data Management applications (EDC, RTSM and ePRO) as well as Clinical Trial Management applications (CTMS and eTMF) that are available for deployment in the cloud or on premises.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.
