Ennov QMS
Quality Management System
Take control of GxP operations with Ennov Process
- Gain full control of your Quality and GxP processes
- Collaborate effectively with our 100% web-based solution
- Automate workflows to eliminate bottlenecks
- Standardize and eliminate variability
- Local language user interface connects global users
- Pre-configured views, reports and dashboards
The Quality Process Management Challenge
Imagine improving the efficiency of your Quality and GxP processes while ensuring regulatory compliance. Sound impossible? Well the good news is that it is possible thanks to Quality Process Management Software from Ennov.
Regulated companies must control their Quality processes for regulatory compliance reasons, while at the same time, make them as efficient and effective as possible to decrease production times and remain competitive.
Manual, paper-based processes can get bogged down and are very hard to manage, track and control. They are also slow, error prone and notoriously difficult to scale and deploy globally.
If these challenges seem all too familiar, our comprehensive fully-integrated Quality Process Management Software, Ennov QMS, can help streamline your Quality operations, ensure compliance and increase your overall efficiency.
Streamline Quality Processes
Ennov Process allows you to automate your Quality processes and enforce the activities, participants and traceability required by your SOPs using our visual form builder and graphical workflow modeling utilities. Ad hoc processes or those that remain stalled for months are a thing of the past.
The “smart” data form associated with the workflow retains all of the information required at each process step and provides each participant with the information they need, in the proper context, to complete their tasks on time. Managers can monitor any workflow, visualize pending and completed tasks and intervene when tasks become delayed.
Automatic alerts, email notifications and escalations keep your processes on schedule and your business running smoothly.
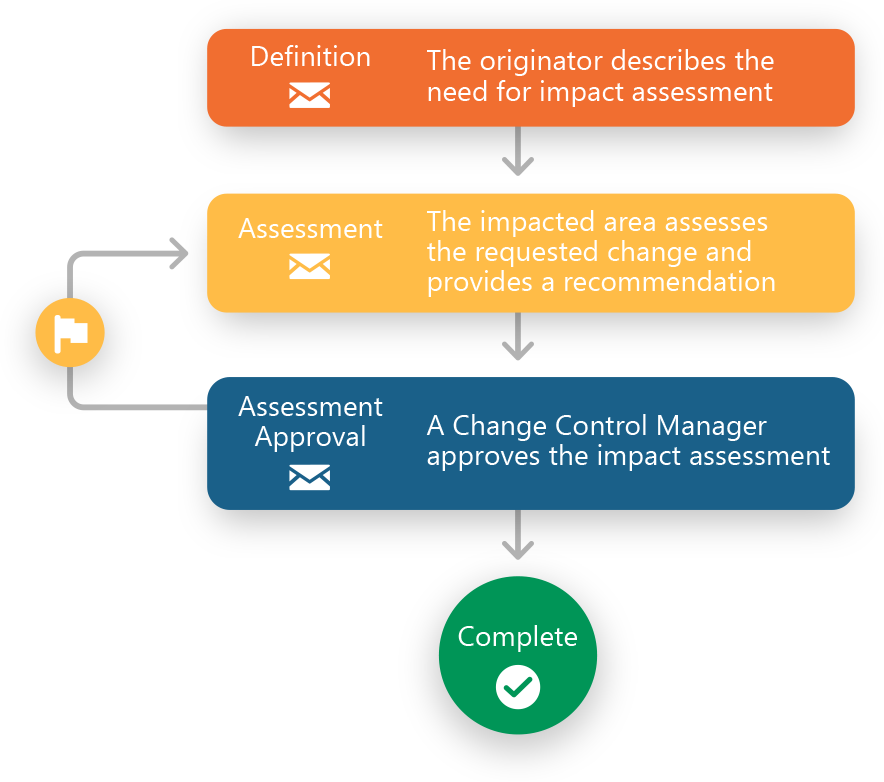
Manage CAPAs, Deviations, Non Conformances, Complaints and more
Our customers use Ennov QMS to support a wide variety of Quality process management needs including CAPAs, Deviations, Non Conformances, Customer Complaints, Change Controls, Audits and more. Ennov QMS’ high degree of configurability and seamless integration with our Enterprise Document Management software (Ennov Doc), our innovative Learning Management software (Ennov Training) and our data visualization and reporting tools (Ennov Analytics) allows them the flexibility to meet their internal Quality standards as well as those of their business partners.
As an added benefit, Ennov QMS fully complies with FDA’s 21 CFR part 11 requirements (electronic signature, audit trail, records management), making it a perfect fit for regulated industries such as pharmaceutical, biotechnology, animal health, medical device and others.
Learn more about :
Core Capabilities
- Graphical workflow designer
- Powerful workflow engine that supports sub-processes
- Visual forms editor
- "Smart" forms that adapt to process step and participant
- Manual or automated task execution
- Real-time monitoring with dashboards and statistics
- Full text and metadata based searching
- REST API for advanced integration
Key Features
- Integrated work list dashboard
- Configurable processes, metadata and views
- Automated email notifications
- Intuitive user interface
- Pre-configured for common quality processes
- Intrinsically connected to Ennov Doc and Ennov Dossier
- 21 CFR Part 11 compliant
- 100% web-based
Pharmacosmos Case Study
“It makes our daily handling and signing of documents much faster, reduces the use of paper and eliminates signing documents by hand.”
Flemming Simonsen,
Director, GxP System Compliance Pharmacosmos
Quality
A Comprehensive QMS to Improve Efficiency and Ensure Compliance
Ennov Quality provides a predefined inventory of quality documentation, processes and workflows that are based on accepted industry standards and best practices. This allows your organization to get your QMS system into production and begin realizing your return on investment more quickly. Ennov Quality, like all Ennov solutions, is easy to configure and requires no IT skills.
A Comprehensive QMS to Improve Efficiency and Ensure Compliance
Ennov Quality provides a predefined inventory of quality documentation, processes and workflows that are based on accepted industry standards and best practices.
This allows your organization to get your QMS system into production and begin realizing your return on investment more quickly. Ennov Quality, like all Ennov solutions, is easy to configure and requires no IT skills.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.
