Ennov Data
Data Management Software
Robust Enterprise Data Management within a Single Repository
Data Management: The Challenges for Regulatory Compliance
In Life Sciences, compliance is no longer just about documents, it’s about data. Structured data now plays a central role across regulatory, clinical, pharmacovigilance, and quality domains.
What began in clinical trials and PV, where data collection and management are foundational, is now essential in regulatory affairs, especially with standards like IDMP. Managing data like product name, dosage, indication, or manufacturer is key to staying compliant and enabling reporting across functions.
That’s why Ennov’s platform is built around a unified, harmonized data model, designed to support complex, cross-domain compliance. With full IDMP implementation, we help you manage the growing demands of data-driven regulation, without system complexity or redundancy.
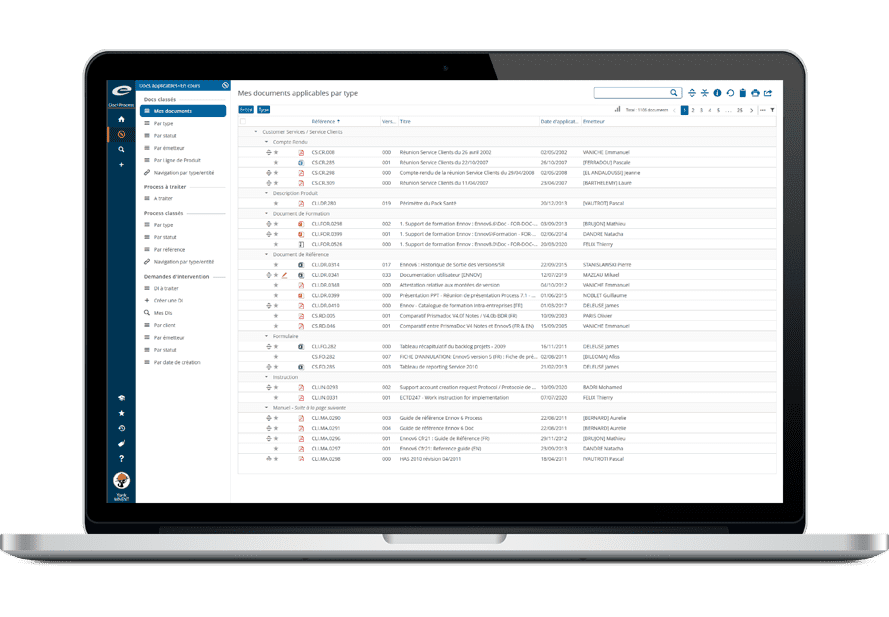
Data Management and Single Repository
The Ennov platform relies on a unified and unique data repository, shared by all modules of the software. Data is stored only once and made available to all services and software that need to use it. This guarantees data homogeneity, as it is no longer necessary to manage silos and reconcile data from multiple sources. All of this contributes to increased confidence in the data within the organization, which has a direct impact on operational performance.
But managing data doesn’t just mean storing it. It also means being able to create associations between data (for example, the molecule and the products it is part of); to manage these associations (what happens if I remove this molecule?); and to control the data update process (who can create or delete data, according to what rules and with what validation). All these actions guarantee data integrity (Data Consistency).
Ennov allows the definition of inheritance rules between objects that are linked to each other in order to reinforce data consistency. The solution also offers strong capabilities to manage the data change process. Thus, Ennov ensures a perfect data consistency, even in the context of the very complex IDMP model.
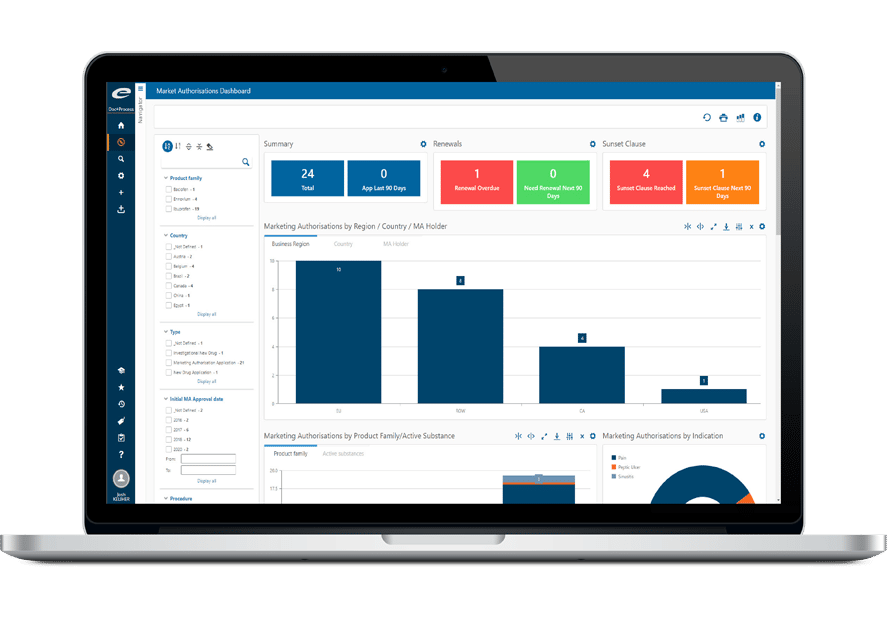
How Does it Relate to Master Data Management?
Data must be shared between different departments, activities and systems. This data can be considered as a central asset of the company, whose control, or lack of control, has direct consequences on its ability to operate effectively in its market. However, as we have also seen, the interactions between data obey extremely complex models.
As a result, Master Data Management projects are essential for many companies in the pharmaceutical sector. The aim is to set up data management systems to ensure that data is consistent and relevant to all the uses to which it can be put within the company. MDM solutions define, data by data, which system is the master, which system is the slave, and how the data are linked together.
Ennov will be able to rely on an MDM when it is in place, in order to provide or consume data. When there is no MDM, Ennov’s RIM can perform this function.
Core Capabilities
- Single Unified Repository & data mart
- Reusable objects linked without integration
- Advanced life cycle management
- Flexible rights management
- Consistent integration with other systems
Key Features
- Intuitive user interface
- Natively designed for IDMP and xEVMPD
- Composite document support
- 21 CFR Part 11 compliant
- 100% web-based
Come join more than 450 Life Sciences companies around the world being powered by Ennov
Ennov Platform
Ennov solutions are elegantly designed to work individually or to be combined together seamlessly for even more efficiency. Built on our Unified Compliance Platform and made specifically for the management of regulated content and processes, each solution is highly configurable, powerful, and easy-to-use. The Ennov platform is the foundation for:
- Regulatory
- Quality
- Commercial
- Clinical
- Pharmacovigilance
Ennov Platform
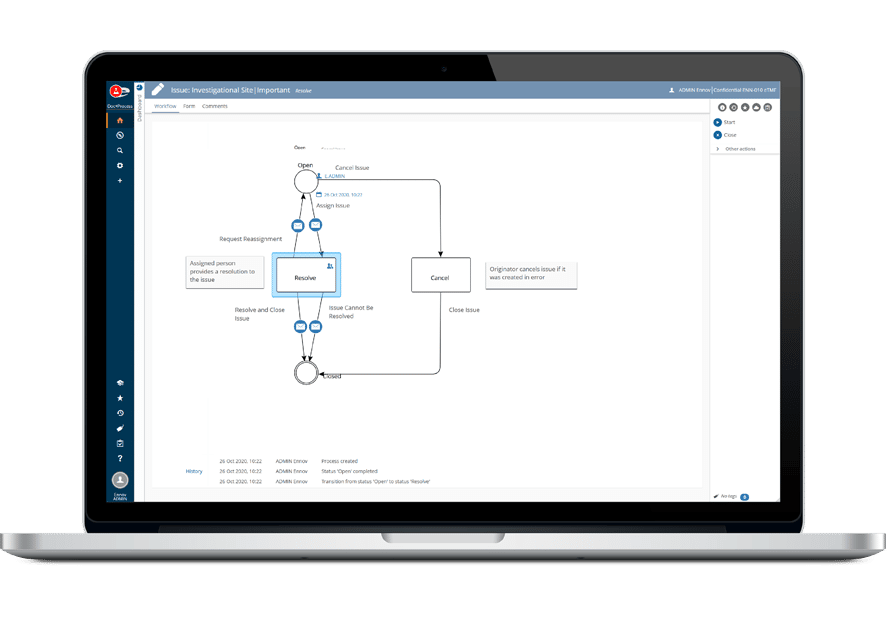
Ennov’s unified compliance platform is a highly configurable software architecture that combines business process management, document management, data/forms management, learning management and business intelligence to form the foundation on which our applications are constructed.
Ennov Platform
Ennov’s unified compliance platform is a highly configurable software architecture that combines business process management, document management, data/forms management, learning management and business intelligence to form the foundation on which our applications are constructed.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment You can switch between deployment options at any time.
- We make you autonomous System configuration and management require no IT skills.
- Improved security and optimized performance Data is hosted locally for total flexibility. Single-tenancy minimizes business interruptions.